In Vitro Fertilization (IVF) and Embryo Cryopreservation
November 4, 2025
Introduction
In Vitro Fertilization (IVF) has become an established treatment for many forms of
infertility and fertility preservation. The usual goal of IVF is to enable a patient to become pregnant using their own or donor eggs and their partner’s or donor sperm. Certain patients will require the participation of a gestational carrier (surrogate). IVF is an elective procedure designed to result in the patient’s pregnancy when other treatments are unsuccessful or are not appropriate. It is also an effective method of fertility preservation.
This consent reviews the entire IVF process, including the risks that this treatment might pose to you and your offspring. The medical risks of IVF vary with respect to each specific step of the procedure. In addition to these known risks, there may be risks with IVF that have not yet been established or even suspected.
An IVF cycle typically includes the following steps or procedures:
- Priming
- Stimulation
- Monitoring
- Egg Retrieval
- Fertilization
- Standard IVF
- Intracytoplasmic sperm injection (ICSI)
- Embryo Culture
- Embryo Cryopreservation (freezing)
Patients are not guaranteed success at any or all of these steps. If optimal results are not achieved at any step, it may be recommended that the treatment be stopped, and the cycle cancelled.
Priming
Some IVF protocols start with a preparation month called “priming.” This often involves taking hormones 2-4 weeks before you begin your stimulation medications. Priming can help suppress your ovaries, help prevent cysts, and optimize scheduling.
Medications (not limited to);
- Oral contraceptive pills:
- Estrogen only
- Estrogen/Progesterone
- Estrogen/Progesterone/Testosterone
These medications can be prescribed by oral, trans-dermal, intramuscular, or vaginal administration.
Risks and Side Effects (not limited to);
Unscheduled bleeding, headache, breast tenderness, nausea, swelling, depression, sleepiness, allergic reaction, and the risk of blood clots or stroke.
If given by injection, includes the additional risk of infection or pain at the injection site.
Stimulation
After your priming month, you will take injectable medications (gonadotrophins) to stimulate your ovaries to produce and grow more mature eggs. Most people need to take these injections every day for 9 to 12 days. Additional injectable medications (GnRH Antagonists, GnRH Agonists) are used to prevent a premature LH surge, which could result in the release of eggs before they are ready to be retrieved. The use of injectable gonadotrophins requires careful monitoring in order to avoid either inadequate or excessive response.
Medications including (but not limited to);
*IVF care plans are personalized. Therefore, not all the drugs on this list apply to you.
Stimulation medications
a. Gonadotrophins
- FSH (Gonal-F®, Puregon®)
- LH (Luveris®)
- Human Menopausal gonadotrophins (Menopur®)
All injectable fertility drugs have FSH (follicle stimulating hormone), a hormone that will stimulate the growth of your ovarian follicles (which contain the eggs). Some of them also contain LH (luteinizing hormone) or LH-like activity. LH is a hormone that may work with FSH to increase the production of estrogen and growth of the follicles. Low-dose hCG (human chorionic gonadotropin) can be used in lieu of LH. These medications are given by subcutaneous or intramuscular injection. Proper dosage of these drugs and the timing of egg recovery requires monitoring of the ovarian response, usually by way of blood tests and ultrasound examinations during the ovarian stimulation.
As with all injectable medications, bruising, redness, swelling, or discomfort can occur at the injection site. Rarely, there can be an allergic reaction to these drugs. The intent of giving these medications is to mature multiple follicles. Many women experience some bloating and minor discomfort as the follicles grow and the ovaries become temporarily enlarged. Up to 2% of women will develop Ovarian Hyperstimulation Syndrome (OHSS) [see full discussion of OHSS in the “Risks to the Patient” section that follows]. Other risks and side effects of gonadotrophins include, but are not limited to, fatigue, headaches, weight gain, mood swings, nausea, and clots in blood vessels.
Even with pre-treatment attempts to assess response, and even more so with abnormal pre-treatment evaluations of ovarian reserve, the stimulation may result in very few follicles developing. The end result may be few or no eggs obtained at egg retrieval. If the stimulation has resulted in a low number of follicles, the patient, in consultation with the POLLIN PHYSICIAN, may choose to cancel the treatment cycle prior to egg retrieval.
Concerns have been raised that the risk of ovarian cancer may increase with the use of fertility drugs, but recent studies have not confirmed this. One risk factor for ovarian cancer is infertility, and early reports may have falsely attributed the risk resulting from infertility to the use of medications to overcome it.
b. Oral Hormones
- Clomiphene Citrate (Clomid®, Serophene®)
- Letrozole (Femara®)
These medications are hormones that are taken orally to stimulate the ovaries to produce more eggs.
LH surge Prevention
a. GnRH Antagonist
- Centrorelix (Cetrotide®)
- Ganirelix (Orgalutran®)
GnRH antagonists are medications that reversibly bind to GnRH receptors in the pituitary gland and prevent release of FSH and LH. These are a class of medications used to prevent premature ovulation prior to egg retrieval. They tend to be used for short periods of time in the late stages of ovarian stimulation. The potential side effects include, but are not limited to: abdominal pain, headaches, skin reaction at the injection site, and nausea.
b. GnRH Agonist
- Buserelin (Superfact®)
- Triptorelin (Decapetyl®)
- Leuprolide Acetate (Lupron®)
These are another class of medications used to prevent premature ovulation prior to egg retrieval. Since GnRH agonists initially cause a release of FSH and LH from the pituitary, they can also be used to start the growth of the follicles or initiate the final stages of egg maturation. Though Leuprolide Acetate is an FDA (U.S. Food and Drug Administration) approved medication, it has not been approved for use in IVF, although it has routinely been used in this way for more than 20 years. Potential side effects usually experienced with long-term use include, but are not limited to: hot flashes, vaginal dryness, bone loss, nausea, vomiting, skin reactions at the injection site, fluid retention, muscle aches, headaches, and depression. No long term or serious side effects are known. Since GnRH agonists are often administered after ovulation, it is possible that they will be taken early in pregnancy. The safest course of action is to use a barrier method of contraception (condoms or oral contraceptives) the month you will be starting these medications. GnRH agonists have not been associated with any fetal malformations, however you should discontinue use of these medications immediately if pregnancy is confirmed.
Trigger
Once your follicles have developed to an appropriate size and number, you will be “triggered” with one or a series of medications that helps the developing eggs complete their maturation process. The timing of this medication is very important.
- Human Chorionic Gonadotropin (hCG)
- Recombinant Human Chorionic Gonadotropin (Ovidrel®)
- Urine-derived Human Chorionic Gonadotropin (Pregnyl®)
hCG is a natural hormone used in IVF to induce the eggs to become mature and fertilizable. The timing of this medication is critical to retrieve mature eggs. Potential side effects include, but are not limited to: breast tenderness, bloating, and pelvic discomfort.
- GnRH Agonist
- Buserelin (Superfact®)
- Triptorelin (Decapetyl®)
- Leuprolide Acetate (Lupron®)
The primary role of these medication is to prevent a premature LH surge, which could result in the release of eggs before they are ready to be retrieved. Since GnRH agonists initially cause a release of FSH and LH from the pituitary, they can also be used to start the growth of the follicles or initiate the final stages of egg maturation prior to egg retrieval. It is this latter property that makes these medications a good trigger option.
Other medications
Antibiotics may be given for a short time during the treatment cycle to reduce the risk of infection associated with egg retrieval or embryo transfer. Antibiotic use may be associated with vaginal yeast infection, nausea, vomiting, diarrhea, rashes, sensitivity to the sun, and allergic reactions. Anti-anxiety medications or muscle relaxants may be recommended prior to the embryo transfer. The most common side effect is drowsiness. Other medications such as steroids, heparin, or aspirin may also be included in the treatment protocol and may be associated with side effects such as bone thinning (steroids), injection site reactions such as redness, irritation and bruising (heparin), and prolonged bleeding (heparin, aspirin).
Note: Many of the medications that are used are administered by an injection. The patient or another person can be instructed to give these injections. Self-administration of medication training will be provided. If you have any questions regarding the drugs or their administration you should ask your IVF team.
Monitoring
While undergoing ovarian stimulation, you will do blood tests and internal (transvaginal) ultrasounds to track the development of the follicles in your ovaries. Drawing of blood samples during this monitoring may carry a risk of mild discomfort and bruising at the blood draw site. Transvaginal ultrasound examinations will be performed, as necessary, and there may be some discomfort with this procedure. While taking the fertility medications, you will need to see us about 4-8 times. These appointments are scheduled early in the morning. You may be asked to return every one to two days until you are ready for the egg retrieval procedure. Missed bloodwork and ultrasound appointments during your ovarian stimulation may impact the success of your cycle.
Egg Retrieval
Oocyte (egg) retrieval is the removal of eggs from the ovary. A transvaginal ultrasound probe is used to visualize the ovaries and the egg-containing follicles within the ovaries. A long needle, which can be seen on ultrasound, can be guided into each follicle and the contents aspirated. The aspirated material includes follicular fluid, oocytes and granulosa (egg-supporting) cells.
Rarely the ovaries are not accessible by the transvaginal route and transabdominal retrieval is necessary. These procedures and risks will be discussed with you by your doctor if applicable. The doctor will give you sedative medications through an IV before the procedure begins to help relax you and provide pain relief. While you will not be asleep, you should be comfortable. Some women may experience some discomfort during the procedure. The procedure takes about 15 to 20 minutes. Afterwards, you will stay in a recovery area for about 1 hour before going home.
Risks of oocyte retrieval include but are not limited to:
Infection: Bacteria normally present in the vagina may be inadvertently transferred into the abdominal cavity by the needle. These bacteria may cause an infection of the uterus, fallopian tubes, ovaries or other intra-abdominal organs. The estimated incidence of infection after egg retrieval is less than 0.5%. Treatment of infections may require the use of oral or intravenous antibiotics. Severe infections occasionally require surgery to remove infected tissue. Infections can have a negative impact on future fertility. Prophylactic antibiotics are sometimes used before the egg retrieval procedure to reduce the risk of pelvic or abdominal infection in patients at higher risk of this complication. Despite the use of antibiotics, there is no way to eliminate this risk completely.
Bleeding: The needle passes through the vaginal wall and into the ovary to obtain the eggs. Both of these structures contain blood vessels. In addition, there are other blood vessels nearby. Small amounts of blood loss are common during egg retrievals. The incidence of major bleeding problems has been estimated to be less than 0.1%. Major bleeding will frequently require surgical repair and possibly loss of the ovary. The need for blood transfusion is rare. (Although very rare, review of the world experience with IVF indicates that unrecognized bleeding has lead to death.)
Trauma: Despite the use of ultrasound guidance, it is possible to damage other intra-abdominal organs during the egg retrieval. Previous reports in the medical literature have noted damage to the bowel, appendix, bladder, ureters, and ovary. Damage to internal organs may result in the need for additional treatment such as surgery to repair or remove the damaged organ. However, the risk of such trauma is low.
Failure: It is possible that the one or more follicles will not allow aspiration (location or proximity to blood vessels), aspiration will fail to obtain any eggs, or the eggs may be abnormal or of poor quality and otherwise fail to produce a viable pregnancy.
Anesthesia: For the egg retrieval, medications usually are administered by an anesthesiologist. The patient will have a consultation with the anesthesiologist before the procedure to review the risks and benefits of the anesthesia. In some cases the use of anesthesia on a specific patient may be associated with an increased risk. In such cases the POLLIN PHYSICIAN may offer local anesthesia without the assistance of an anesthesiologist. It is mandatory that there is no oral intake after midnight the evening prior to the egg retrieval. After the procedure is completed, the patient will be discharged home usually within one hour. Because of the anesthetic medications that are used, a patient must be accompanied home by a responsible adult. Each patient is responsible for bringing a responsible adult with them on the day of the egg retrieval. Following the egg retrieval, vaginal spotting and lower abdominal cramping are normal. During the remainder of the day following the surgery, activities should be limited. If significant bleeding, vomiting, abdominal pain or any other symptoms develop, you should contact your POLLIN PHYSICIAN. If you should have any difficulty contacting your POLLIN PHYSICIAN, you or your caretaker should proceed to the emergency department of the nearest hospital.
Fertilization of the Eggs
After eggs are retrieved, they are transferred to the embryology laboratory where they are kept in conditions that support their growth. The eggs are placed in small dishes or tubes containing “culture media,” (special fluid that resembles the fluid found in the fallopian tube or uterus) that supports embryo development.
Our goal is to attempt to fertilize all eggs retrieved, unless you have discussed an alternative with your POLLIN PHYSICIAN and signed appropriate forms indicating your wishes. A few hours after eggs are retrieved, sperm are either placed in the culture medium with the eggs (standard IVF), or individual sperm are injected into each mature egg in a technique called Intracytoplasmic Sperm Injection (ICSI). The eggs are then returned to the incubator to develop. The following day after eggs have been inseminated or injected with a single sperm (ICSI), they are examined for signs that the process of fertilization is underway. At this stage, normal development is evident by the still single cell having 2 nuclei; this stage is called a zygote or a 2PN embryo.
Under some circumstances, it is recommended to freeze sperm prior to the day of egg retrieval for use on the day of egg retrieval. Reasons to consider sperm freezing would be if the sperm provider may not be available on the day of the egg retrieval or there has been difficulty in the past with the production of a semen sample. You are responsible for making arrangements to freeze sperm prior to the start of treatment if this applies to you. The source of the sperm can be from the sperm provider or in some situations the couple or patient may choose to use donor sperm. The biologist processes the sperm sample and then the eggs are inseminated. There are two approaches to the insemination of the eggs:
a. Standard IVF: After the sperm sample has been processed, a mixture of the sperm and eggs is placed in a plastic dish containing a nutrient-rich culture media and then placed in an incubator in the laboratory to allow fertilization to occur. The nutrient-rich culture media contains a serum additive, which is a blood product, and there is a rare chance of transmission of a viral infection. The morning after the egg retrieval, the eggs are examined to see if fertilization has occurred.
POLLIN FERTILITY recommends that you review the ICSI information in the IVF Information Package and consent to ICSI, even if ICSI is not planned. In some cases, ICSI may be unexpectedly required to improve the chances of pregnancy in the cycle.
b. Intracytoplasmic sperm injection (ICSI) - ICSI is a laboratory procedure performed to increase the chances of fertilization. The ICSI procedure is a process whereby, with the aid of a microscope and fine instruments, a single sperm is injected directly into the egg. Indications for ICSI include a previous IVF cycle with poor fertilization, a previous semen analysis demonstrating abnormalities, and in situations where surgical aspiration of sperm from the vas deferens or testicle is required. However, given the increased rate of fertilization associated with ICSI as compared to standard IVF, ICSI is often the preferred choice of egg fertilization for most patients undergoing IVF. In most cases it is known at the start of the IVF cycle that ICSI will be performed. However, in other cases, the sperm sample on the day of the egg retrieval may be unexpectedly inadequate for standard insemination and the ICSI procedure may be performed.
Research investigating the potential risks associated with ICSI as compared to standard IVF have yielded conflicting results and, overall, appear to support ICSI as a safe method of fertilization. Although one study identified an association between ICSI and certain congenital anomalies, the overall risk was low and only marginally increased relative to children conceived naturally (4.2% versus ~3% of those conceived naturally). In addition, it was unclear whether this difference was due to the ICSI procedure itself or inherent sperm defects. Similarly, one study identified an increase in the prevalence of sex chromosome (X and Y) abnormalities in children conceived via ICSI as compared to standard IVF but this risk was also extremely low (0.8-1.0% in ICSI offspring vs. 0.2% in the general IVF population) and, again, it was not possible to determine whether this difference was related to the ICSI procedure itself or the underlying cause of male factor infertility.
Some causes of severe male factor infertility are genetic in nature and require the surgical extraction of sperm from the testicle or epididymis. All surgically extracted sperm requires ICSI for egg fertilization. Although this allows men who would have otherwise been unable to suse their sperm to achieve a pregnancy, it can increase the risk of transmission of certain genetic conditions associated with infertility to the offspring. Genetic testing options such as carrier screening and preimplantation genetic testing can reduce the risks of these conditions occurring in the offspring. If you would like additional information about the genetic issues surrounding IVF and the ICSI procedure talk to your physician about a referral to a genetics counselor.
The following additional risks are associated with the performance of the ICSI procedure:
- The eggs may fail to become fertilized or may be damaged precluding their ability to be fertilized.
- ICSI may yield presently unknown risks to the baby and/or mother.
- Studies have shown that some cases of male infertility may be genetic. Therefore, there is the possibility that infertility may be passed on to the offspring as stated above. Some studies show an increased risk of certain congenital and chromosomal abnormalities in babies born as a result of the ICSI procedure. If pregnancy is achieved testing can be performed to determine the chromosomal makeup of the fetus. If you would like additional information concerning genetics and inheritance, you should ask your POLLIN PHYSICIAN to refer you to a genetic counselor prior to the start of your treatment cycle.
On average, 60-70% of eggs will fertilize following the standard insemination or the ICSI procedure but in some cases none of the eggs fertilize. If fertilization is confirmed, plans are then made for the embryo transfer. In some cases of documented fertilization the embryos stop their development and the embryo transfer is cancelled.
Embryo Culture
After fertilization, the embryos (eggs fertilized with sperm) are then returned to the incubator to develop. Periodically over the next few days, the dishes are inspected so the development of the embryos can be monitored.
Day 1: Normal development is evident by the still single cell having 2 nuclei; this stage is called a zygote or a 2PN embryo.
Day 2: Normal embryos have divided into about 4 cells.
Day 3: Normally developing embryos contain about 8 cells.
Day 5-7: Normally developing embryos have developed to the blastocyst stage, which is typified by an embryo that now has 80 or more cells, an inner fluid-filled cavity, a small cluster of cells called the inner cell mass, and an outer group of cells called the trophectoderm.
It is important to note that since many eggs and embryos are abnormal, it is expected that not all eggs will fertilize and not all embryos will divide at a normal rate. The chance that a developing embryo will produce a pregnancy is related to many factors including whether its development in the lab is normal, but this correlation is not perfect. This means that not all embryos developing at the normal rate are in fact also genetically normal, and not all poorly developing embryos are genetically abnormal. Nonetheless, their visual appearance is the most common and useful guide in the selection of the best embryo(s) for transfer.
Time-lapse
During the first 3 days of embryo culture after fertilization, POLLIN FERTILITY may use an embryo imaging system called Time Lapse Imaging (TLI) within our incubators that captures key events during early development. TLI allows continuous monitoring of embryo development without disturbing culture conditions by removing embryos from the incubator. Information from these automated cell division measurements are used to enhance the ability to select the most viable embryo from the cohort and provide some information about the embryo’s reproductive potential. Long-term effects on the embryos of this imaging are unknown but the amount of additional light exposure to the embryos is minimal and equivalent to approximately the same or less amount of light that an embryo is exposed to during traditional evaluations outside of the incubator.
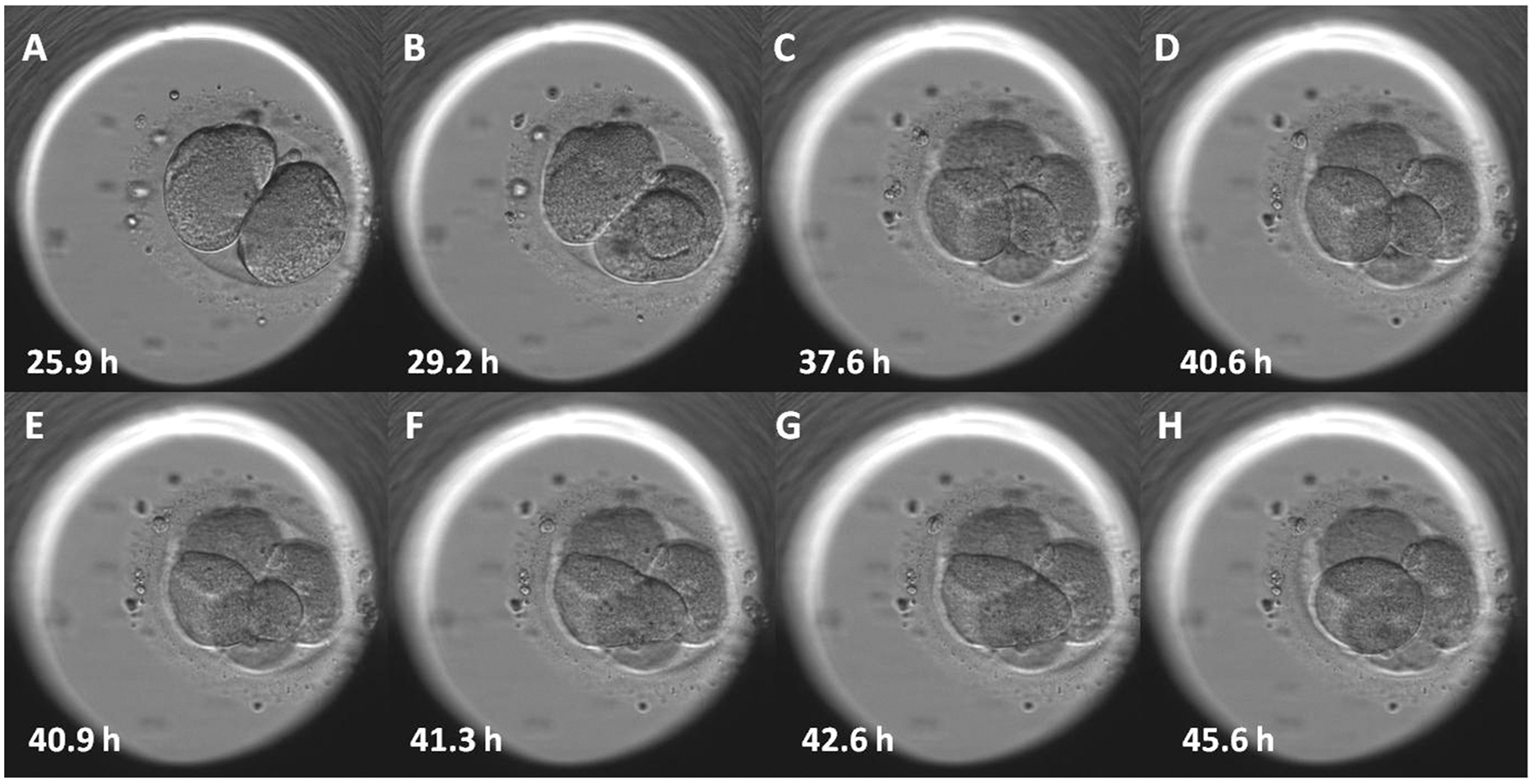
Although every reasonable precaution is taken, any of the following may occur in the lab:
- Fertilization of the egg(s) may fail to occur.
- One or more eggs may be fertilized abnormally resulting in an abnormal number of chromosomes in the embryo. Abnormal embryos identified as having chromosomal abnormalities will not be transferred.
- The fertilized eggs may degenerate before dividing into embryos, or adequate embryonic development may fail to occur.
- Bacterial contamination or a laboratory accident may result in loss or damage to some or all of the eggs or embryos.
- Laboratory risks (see comprehensive list on pg 22)
- Other unforeseen circumstances may prevent the performances of any step of the procedure, or prevent the establishment of a pregnancy.
- Hurricanes, floods, or other ‘acts of God’ (including bombings or other terrorist acts) could destroy the laboratory or its contents, including sperm, eggs, or embryos being stored.
Freezing (Cryopreservation) of Embryos
Freezing (or cryopreservation) of eggs or embryos is a common procedure. Since multiple eggs (oocytes) are often produced during ovarian stimulation, there may be more embryos available than are considered appropriate for transfer to the uterus. Such embryos can be frozen for future use. Furthermore, the availability of cryopreservation permits patients to transfer fewer embryos during a fresh cycle, reducing the risk of high-order multiple gestations (triplets or greater). Other possible reasons for cryopreservation of embryos include freezing all embryos in the initial cycle to prevent severe ovarian hyperstimulation syndrome (OHSS), if there is a greater chance of pregnancy expected in a frozen cycle, if genetic testing is being performed with no results available in time for a fresh transfer, if a patient or couple were concerned that their future fertility potential might be reduced due to necessary medical treatment (e.g., cancer therapy or surgery), or for personal reasons.
Embryos that are not of sufficient quality will not be frozen and will be discarded. At a later date frozen embryos can be thawed for transfer without the need for ovulation induction medications or an egg retrieval. If the patient(s) decides later that they no longer want to continue treatment, then the frozen embryos can be thawed and discarded or donated for teaching, research or to a third party in accordance with the patient(s)’ instructions.
Indications for cryopreservation:
- To reduce the risks of multiple gestation
- To preserve fertility potential in the face of certain necessary medical procedures or for personal reasons
- To increase the chance of having one or more pregnancies from a single cycle of ovarian stimulation
- To minimize the medical risk and cost to the patient by decreasing the number of stimulated cycles and egg retrievals
- To temporarily delay pregnancy and decrease the risks of hyperstimulation (OHSS; see below) by freezing all embryos, when this risk is deemed to be high
- To await results of genetic testing
- To increase pregnancy rate if there are concerns about uterine lining in a fresh cycle
Risks of embryo cryopreservation: There are several techniques for embryo cryopreservation and research is ongoing. Traditional methods include “slow,” graduated freezing and “rapid” freezing methods, called “vitrification.” Current techniques deliver a high percentage of viable embryos thawed after cryopreservation, but there can be no certainty that embryos will thaw normally, nor be viable enough to divide and eventually implant in the uterus. Cryopreservation techniques could theoretically be injurious to the embryo. Extensive animal data (through several generations), and limited human data, do not indicate any likelihood that children born of embryos that have been cryopreserved and thawed will experience greater risk of abnormalities than those born of fresh embryos. However, until very large numbers of children have been born following freezing and thawing of embryos, it is not possible to be certain that the rate of abnormalities is no different from the normal rate.
Disposition of unused Embryos and Oocytes
POLLIN FERTILITY wants to provide you with relevant and appropriate information so that you may make an informed and voluntary choice regarding the disposition of any embryos remaining following your treatment (”unused embryos”). Because of the possibility of you and/or your partner’s separation, divorce, death or incapacitation after embryos have been produced, it is important to decide on the disposition of any embryos (fresh or cryopreserved) that remain in the laboratory in these situations. Since this is a rapidly evolving field, both medically and legally, the clinic cannot guarantee what the available or acceptable avenues for disposition will be at any future date. Your physician will discuss with you the following options and you will be required to complete POLLIN FERTILITY’S “Disposition of Embryos” consent form prior to commencing treatment. Please note that Section 8 of the Assisted Human Reproduction Act limits the uses of embryos under certain circumstances (such as the death of one of the intended parents), and clinic consents cannot be used to over-ride the federal legislation.
Options include:
- Storing (cryopreservation) any unused embryo(s)
- Discarding any unused embryo(s)
- Donating any unused embryo(s) for approved research studies
- Donating any unused embryo(s) for the purpose of quality improvement
- Donating any unused embryos to another individual in order to attempt pregnancy.
- In the event of the death of one of the partners who contributed gametes to the creation of the embryos, use by the surviving partner with the intention of initiating a pregnancy provided that as of the date of death, the partners were still spouses or common-law partners. POLLIN FERTILITY may require proof of the status of the relationship as of the date of death, and each partner’s choice may be withdrawn at any time prior to the date of death, by providing notice in writing to POLLIN FERTILITY and the POLLIN PHYSICIAN.
Discard: Unused embryos or oocytes can be ethically discarded in accordance with current institutional policies. The unused embryos or oocytes will no longer be available for attempting pregnancy.
Research: Donated embryos and oocytes may be used by researchers interested in the study of human reproduction or development or human embryonic stem cell research. By initialing this choice, you may be contacted by a POLLIN FERTILITY research coordinator who will provide additional information and/or a separate research consent form so that you can consent to the use of the embryos in a specific research project.
Quality improvement: Donated embryos and oocytes may be used in ongoing efforts to develop and improve IVF techniques, train staff, and conduct quality control.
Disposition of Clinically Unsuitable or non-selected Mterials
Embryos which result from abnormal fertilization (i.e., polyspermy - when more than one sperm fertilizes an egg) will be discarded because they have no chance of developing normally. In addition, embryos that fail to develop properly (e.g., fail to divide, demonstrate other significant abnormalities of development) will also be discarded. Eggs and/or embryos, which have failed to develop (not viable), will not be transferred and will be discarded.
In most cycles, some eggs retrieved during the in vitro fertilization process are immature, fail to fertilize, or are abnormally fertilized and incapable of further development. Some embryos are determined to be nonviable because they stop developing in vitro. These eggs and embryos that are unsuitable for transfer, which would normally be discarded, could be of use to our clinical team in undertaking quality control, improving IVF techniques, or to researchers interested in the study of human reproduction or development or human embryonic stem cell research. The determination that an egg or embryo is unsuitable for clinical use will be made by staff embryologists who are not performing the research. In addition, in cycles in which Preimplantation Genetic Testing (PGT) is performed, PGT embryos that you and your POLLIN PHYSICIAN agree will not be used for your care (due to abnormality or other documented reason) may be donated to research or quality improvement techniques, or discarded, in accordance with your choice.
In certain situations, donating materials to research may not be possible; in this instance, your materials will be ethically discarded in accordance with current institutional policies.
Deciding that you do not want to donate to research or technique improvement will have no effect on your future care at POLLIN FERTILITY. Even if you do decide now that you would like to donate, you may change your mind by notifying POLLIN FERTILITY, in writing, at any time prior to the use of your embryo.
All patients are required to complete the Disposition of Eggs or Disposition of Embryos consent form, as applicable, prior to oocyte retrieval. That form outlines the choices you have with regard to the disposition of oocytes and embryos in a variety of situations that may arise. You are free to submit a statement at a later time indicating different choices, provided PATIENT and, where applicable, your PARTNER agree in writing. It is also incumbent upon you to remain in touch with POLLIN FERTILITY regarding your contact information, and to pay for storage charges as they come due.
Options for clinically unsuitable or non-selected materials include:
1. Discarding any unused materials
2. Donating any unused materials for approved research studies
3. Donating any unused materials for the purpose of quality improvement
Discard: Unused embryos or oocytes can be ethically discarded in accordance with current institutional policies. The unused embryos or oocytes will no longer be available for attempting pregnancy.
Research: Donated embryos and oocytes may be used by researchers interested in the study of human reproduction or development or human embryonic stem cell research. By initialing this choice, you may be contacted by a POLLIN FERTILITY research coordinator who will provide additional information and/or a separate research consent form.
Quality improvement: Donated embryos and oocytes may be used in ongoing efforts to develop and improve IVF techniques, train staff and conduct quality control
Risks to the Patient
1. Ovarian Hyperstimulation Syndrome
To increase the number of eggs that develop, a series of hormone injections are given. These hormones are known to have a variety of side effects, some minor and some potentially major. The most serious side effect of ovarian stimulation is ovarian hyperstimulation syndrome (OHSS). Its symptoms can include increased ovarian size, nausea and vomiting, accumulation of fluid in the abdomen, breathing difficulties, an increased concentration of red blood cells, kidney and liver problems, and in the most severe cases, blood clots, kidney failure, or death. The severe cases affect only a very small percentage of women who undergo in vitro fertilization - 0.2 percent or less of all treatment cycles - and the very severe are an even smaller percentage. Only about 1.4 in 100,000 cycles has lead to kidney failure, for example. OHSS occurs at two stages: early, 1 to 5 days after egg retrieval (as a result of the hCG trigger); and late, 10 to 15 days after retrieval (as a result of the hCG hormone if pregnancy occurs). The risk of severe complications is about 4-12 times higher if pregnancy occurs which is why sometimes no embryo transfer is performed to reduce the possibility of this occurring. IVF protocols exist that significantly reduce the risk of OHSS.
2. Injections
As with all injectable medications, bruising, redness, swelling, or discomfort can occur at the injection site. Rarely, there can be an allergic reaction to these drugs. Fertility medications can cause side effects such as nausea, vomiting, hot flashes, headaches, mood swings, visual symptoms, memory difficulties, joint problems, weight gain and weight loss, all of which are temporary. The intent of giving these medications is to mature multiple follicles, and many women experience some bloating and minor discomfort as the follicles grow and the ovaries become temporarily enlarged.
3. Ovarian Torsion (Twisting)
In less than 1% of cases, a fluid filled cyst(s) in the ovary can cause the ovary to twist on itself. This can decrease the blood supply to the ovary and result in significant lower abdominal pain. Surgery may be required to untwist or possibly remove the ovary.
4. Cancer
Many have worried that the use of fertility drugs could lead to an increased risk of cancer, in particular, breast, ovarian, and uterine cancers. One must be careful in interpreting epidemiological studies of women taking fertility drugs, because all of these cancers are more common in women with infertility, so merely comparing women taking fertility drugs with women in the general population inevitably shows an increased incidence of cancer. When the analysis takes into account the increased cancer risk due to infertility per se, the evidence does not support a relationship between fertility drugs and an increased prevalence of breast or ovarian cancer. More research is required to examine what the long-term impact of fertility drugs may be on breast and ovarian cancer prevalence rates. For uterine cancer, the numbers are too small to achieve statistical significance, but it is at least possible that use of fertility drugs may indeed cause some increased risk of uterine cancer.
5. Risks of Pregnancy
Pregnancies that occur with IVF are associated with increased risks of certain conditions in the woman carrying the pregnancy including pre-eclampsia, placenta previa, placental abruption, gestational diabetes, and cesarean section. Some of these risks stem from the higher average age of women undergoing IVF, and the fact that the underlying cause of infertility may be an independent cause of the increased risk of pregnancy complications. Others risks include, but are not limited to:
- Miscarriage - The risk of miscarriage in the general population is 15-20%. The risk of miscarriage increases with the age of the patient and for patients over 40 years of age, the risk may exceed 50%. Studies have shown either no increase or a slight increase in the risk of miscarriage in patients who conceive with this treatment. Most miscarriages are associated with lower abdominal cramping and bleeding, but do not necessarily require treatment. In some cases, however, complete removal of the pregnancy tissue must be accomplished by a surgical procedure called a dilatation and curettage (D&C) or dilatation and evacuation (D&E). This procedure is usually performed under anesthesia in the operating room and involves placing a suction tube into the uterine cavity to remove the pregnancy tissue.
- Chemical Pregnancy - A chemical pregnancy is a type of miscarriage that occurs when a fertilized egg starts to implant in the uterus, leading to a positive pregnancy test, but does not continue to develop into a pregnancy that can be seen on an ultrasound. Chemical pregnancies often occur so early that some patients do not realize they have happened. Chemical pregnancies do not require any specific treatment.
- Tubal (Ectopic) Pregnancy - An ectopic pregnancy is an abnormal pregnancy that develops outside of the uterus. The majority of ectopic pregnancies occur in the fallopian tube. The risk of tubal pregnancy is approximately 2% and is greater in a woman with damaged tubes. If a patient has a tubal pregnancy, they may need surgical treatment, which may involve the removal of the involved tube. Medical treatment with methotrexate may be an option in selected cases.
6. Multiple Gestation
Multiple gestations are a known risk of pregnancies achieved through IVF and substantially increase the risk of pregnancy complications. The most important maternal complications associated with multiple gestations are preterm labor and delivery, preeclampsia, and gestational diabetes. Placenta previa (placenta extends over the cervical opening), vasa previa (one or more of the blood vessels extends over the cervical opening), and placental abruption (premature separation of the placenta) are also more common in multiple gestations. Postpartum hemorrhage may complicate 12% of multifetal deliveries. Having triplets or more increases the risk of more significant complications including post-partum hemorrhage and transfusion. Other complications of multiple gestations include gallbladder problems, skin problems, excess weight gain, anemia, excessive nausea and vomiting, and exacerbation of pregnancy-associated gastrointestinal symptoms.
6. Failed Cycle
There are numerous reasons why a cycle may be unsuccessful including, but not limited to, poor or unusual response to medications, no eggs present at the time of egg retrieval, no fertilization of the egg with sperm, and inadequate embryo quality to allow for transfer or freezing (see “Unknowns” section below for more detail).
7. Psychosocial Effects of Infertility Treatment
Infertility and its treatment can affect your emotions, your health, your finances, and your social life. Treatment is time-consuming and may strain your personal relationships and your religious or ethical beliefs. During treatment, you may feel anxious, helpless, depressed, or all alone. You may go through highs and lows. The outcome may not be what you want as a pregnancy cannot be guaranteed. In some cases, you may want to seek the help of a mental health expert to help you through the emotional implications of treatment. Your clinic can provide resources to appropriate mental health professionals in your area.
Risks to Offspring
1. Overall Risks
Since the first birth of an IVF baby in 1978, more than 3 million children have been born worldwide following IVF treatments. Numerous studies have been conducted to assess the overall health of IVF children and the majority of studies on the safety of IVF have been reassuring. As more time has passed and the dataset has enlarged, some studies have raised doubts about the equivalence of risks for IVF babies as compared to naturally conceived babies. A major problem in interpreting the data arises from the fact that comparing a group of infertile couples to a group of normally fertile couples is not the proper comparison to make if one wants to assess the risk that IVF technology poses. Infertile couples, by definition, do not have normal reproductive function and might be expected to have babies with more abnormalities than a group of normally fertile couples. This said, even if the studies suggesting an increased risk to babies born after IVF prove to be true, the absolute risk of any abnormal outcome appears to be small. Singletons conceived with IVF tend to be born slightly earlier than naturally conceived babies (39.1 weeks as compared to 39.5 weeks). IVF twins are not born earlier or later than naturally conceived twins. The risk of a singleton IVF conceived baby being born with a birth weight under 5 pounds nine ounces (2500 grams) is 12.5% vs. 7% in naturally conceived singletons.
2. Birth Defects
The risk of birth defects in the normal population is 2-3%. In IVF babies the birth defect rate may be 2.6-3.9%. Studies to date have not been large enough to prove a link between IVF treatment and specific types of birth defects.
3. Imprinting Disorders
These are rare disorders having to do with whether a maternal or paternal gene is inappropriately expressed. Since the incidence of this syndrome in the general population is 1/15,000, even if there is a 2-5-fold increase to 2-5/15,000, this absolute risk is very low.
4. Childhood cancers
Most studies have not reported an increased risk with the exception of retinoblastoma and the incidence of this condition is still exceedingly low.
5. Infant Development
In general, studies of long-term developmental outcomes of children born from IVF have been reassuring so far.
6. Risks of a Multiple Pregnancy
Within Canada, 5-10% of IVF pregnancies are twins or higher-order multiple gestations (triplets or greater). Identical twinning occurs in 1.5-4.5% of IVF pregnancies. IVF twins deliver on average three weeks earlier and weigh 1,000 gm (2.2 pounds) less than IVF singletons. Of note, IVF twins do as well as spontaneously conceived twins. Triplet (and greater) pregnancies deliver before 32 weeks (7 months) in almost half of cases.
Prematurity accounts for most of the excess perinatal morbidity and mortality associated with multiple gestations. Moreover, IVF pregnancies are associated with an increased risk of prematurity, independent of maternal age and fetal numbers. Fetal growth problems and discordant growth among the fetuses also result in perinatal morbidity and mortality. Multifetal pregnancy reduction (where one or more fetuses are selectively terminated) reduces, but does not eliminate, the risk of these complications.
Fetal death rates for singleton, twin, and triplet pregnancies are 4.3 per 1,000, 15.5 per 1,000, and 21 per 1,000, respectively. The death of one or more fetuses in a multiple gestation (vanishing twin) is more common in the first trimester and may be observed in up to 25% of pregnancies after IVF. Loss of a fetus in the first trimester is unlikely to adversely affect the surviving fetus or mother. No excess perinatal (mature fetus or newborn) or maternal morbidity has been described resulting from a “vanishing” embryo.
Demise of a single fetus in a twin pregnancy after the first trimester is more common when they share a placenta, ranging in incidence from 0.5-6.8%, and may cause harm to the remaining fetus.
Monozygotic twinning (MZT) is a multiple pregnancy that results from the splitting of a single embryo, which will lead to a set of identical twins. The incidence of MZT is increased in pregnancies conceived following IVF and may occur between 1.5-5% of IVF pregnancies. In addition to the above stated complications associated with a multiple pregnancy with MZT there is a greater chance of twin-to-twin transfusion, which can affect the growth of the fetuses and increase the chance of other complications. Twins sharing the same placenta have a higher frequency of birth defects compared to pregnancies having two placentas. MZT occurs more frequently after blastocyst transfer.
Consequences of multiple gestations include the major sequelae of prematurity (cerebral palsy, retinopathy of prematurity, and chronic lung disease) as well as those of fetal growth restriction (polycythemia, hypoglycemia, necrotizing enterocolitis). It is unclear to what extent multiple gestations themselves affect neuro-behavioral development in the absence of these complications. Rearing of twins and high-order multiples may generate physical, emotional, and financial stresses, and the incidence of maternal depression and anxiety is increased in women raising multiples. At mid-childhood, prematurely born offspring from multiple gestations have lower IQ scores, and multiple birth children have an increase in behavioral problems compared with singletons. It is not clear to what extent these risks are affected by IVF per se.
The Option of Multifetal Pregnancy Reduction: The greater the number of fetuses within the uterus, the greater is the risk for adverse perinatal and maternal outcomes. Patients with more than twins are faced with the options of continuing the pregnancy with all risks previously described, terminating the entire pregnancy, or undergoing a procedure called multifetal pregnancy reduction (MFPR). By reducing the number of fetuses, MFPR decreases risks associated with preterm delivery, but often creates important ethical dilemmas. Pregnancy loss is the main medical risk of MFPR. However, current data suggest that such medical complications have decreased as experience with the procedure has grown. The risk of loss of the entire pregnancy after MFPR is approximately 2-3%, although this risk increases when the number of fetuses prior to the procedure is greater than three.
Unknowns
There are many complex and sometimes unknown factors, which may prevent the establishment of pregnancy. Known factors, which may prevent the establishment of pregnancy, include, but are not limited to, the following:
- The ovaries may not respond adequately to the medications.
- Technical problems including inadequate visualization or the position of the ovaries may prevent the retrieval of the eggs.
- There may be failure to recover an egg because ovulation has occurred prior to the time of the egg retrieval.
- Eggs may not be recovered.
- The eggs may not be normal.
- The male partner, where applicable, may be unable to produce a semen sample or the semen sample may be of insufficient quantity or quality.
- Fertilization of the eggs and sperm to form embryos may not occur.
- Cell division of the embryos may not occur.
- The embryos may not develop normally.
- Embryo transfer into the uterus may be technically difficult or impossible.
- If the transfer is performed, implantation may not result.
- If implantation occurs, the embryo(s) may not grow or develop normally.
- Equipment failure, infection, technical problems, human error and/or unforeseen factors may result in loss or damage to the eggs, semen sample and/or embryos.
The foregoing general information is based upon the experience and knowledge of the POLLIN FERTILITY physicians. It is based, in part, upon a review of the literature pertaining to Reproductive Medicine. This information is generally accurate and comprehensive, however, medicine is a dynamic discipline and reproductive medicine in particular is constantly evolving. Estimates of risks and the relative benefits of alternative treatment that have been discussed with you represent the best professional judgment of the physicians and caregivers of POLLIN FERTILITY taking into account your specific needs and circumstances.
Other Considerations
Ethical and Religious Considerations in Infertility Treatment
Infertility treatment can raise concerns and questions of an ethical or religious nature for some patients.
Psychosocial Effects of Infertility Treatment
IVF can be psychologically stressful. Anxiety and disappointment may occur at any of the phases described above. Significant commitment of time and finances may be required. Couples are encouraged to consider meeting with a counselor. If you are interested in meeting with a social worker or psychologist please speak to your POLLIN PHYSICIAN.
Legal Considerations and Legal Counsel
We understand that both federal and provincial law may impact the use of gametes, creation and use of embryos, the number of possible parents, and the parentage status of any resulting child(ren) conceived with donor gametes or carried by a surrogate. We acknowledge that POLLIN FERTILITY has not give us legal advice, that we are not relying on POLLIN FERTILITY to give us any legal advice, and that we have been informed that we may choose to consult a lawyer who is experienced in the areas of reproductive law if we have any questions or concerns about the present or future status of our embryos, our individual or joint access to them, our individual or joint access to them, our individual or joint parental rights to any resulting child, questions around birth registration, or about any other aspect of this consent and agreement.
Alternatives to IVF
There are alternatives to IVF treatment including the use of donor sperm, donor eggs, adoption or not pursuing treatment are also options. Gametes (sperm and/or eggs), instead of embryos may be frozen for future attempts at pregnancy for a variety of reasons.
Reporting Obligations and Privacy
Data from your ART procedure will also be provided to the Better Outcomes Registry & Network (BORN) and Canadian Assisted Reproductive Technologies Register (CARTR) Databases. Funded by the Government of Ontario, BORN is Ontario's perinatal, newborn and child registry with the role of facilitating quality care for families across the province. BORN collects, interprets, shares and rigorously protects high-quality data essential to making Ontario one of the safest places in the world to have a baby. BORN helps fertility clinics in Canada collect, store, and use fertility treatment data through the CARTR database. Patient information is collected anonymously with no identifying details.
The foregoing general information is based upon the experience and knowledge of the POLLIN FERTILITY physicians. It is based, in part, upon a review of the literature pertaining to Reproductive Medicine. This information is generally accurate and comprehensive, however, medicine is a dynamic discipline and reproductive medicine, in particular, is constantly evolving. Estimates of risk factors and the relative benefits of alternative treatment that have been discussed with you represent the best professional judgement of the physicians and caregivers of POLLIN FERTILITY taking into account your specific needs and circumstances.



